Introduction: B cell activating factor (BAFF) and a proliferation inducing ligand (APRIL) play key roles in B cell development, survival, and differentiation into antibody-secreting cells (ASC). Given their importance in ASC survival and antibody production, inhibition of BAFF and/or APRIL (particularly in combination) is a promising approach for the treatment of a variety of autoimmune or autoantibody-related diseases including autoimmune cytopenias like warm autoimmune hemolytic anemia (wAIHA), immune thrombocytopenia (ITP), and cold agglutinin disease (CAD). Significantly higher levels of both BAFF and APRIL have been observed in the serum of patients with AIHA and ITP compared to healthy subjects, and polymorphisms in BAFF and TACI have been associated with ITP [Abdel-Hamid et al., Am J Med, 2011; Xu et al., Int J Hematol., 2015; Emmerich, et al., Br J Haematol, 2007; Peng, et al., J Thromb Haemost, 2017; Yu, et al., Blood Adv, 2021]. Belimumab, an anti-BAFF antibody, has demonstrated encouraging efficacy in the treatment of ITP associated with systemic lupus erythematosus [De Marchi, et al., Clin Exp Rheumatol, 2017] and in combination with rituximab in patients with ITP [Mahévas, et al., Haematologica, 2021]. Currently, there are no BAFF and/or APRIL inhibitors approved to treat AIHA or ITP. Povetacicept (ALPN-303) is an engineered, potent dual BAFF/APRIL antagonist. We previously evaluated the impact of BAFF/APRIL co-inhibition preclinically in the accelerated HODxOTII transgenic mouse model of AIHA, in which povetacicept treatment significantly suppressed plasma cells in the spleen and bone marrow, reduced pathogenic anti-RBC autoantibodies, and significantly increased hematocrit [Dillon et al., ASH 2022 abstract #3763]. Here, we have evaluated povetacicept in a second preclinical model of AIHA, a rat red blood cell (RRBC)-to-mouse model of erythrocyte autoimmunity.
Methods: In the RRBC model, C57BL/6NJ mice were immunized weekly with 2 x 10 8 Wistar rat RBC for 10 weeks, leading to development of anti-mouse RBC autoantibodies. Groups of mice were treated twice weekly via intraperitoneal injection with povetacicept or a matched Fc isotype control. Analyses included anti-RRBC alloantibodies and autoantibodies bound to mouse erythrocytes (measured via direct anti-globulin test [DAT]). While significant anemia does not occur in this model, serum lactate dehydrogenase (LDH) was also measured as an index measure of subclinical hemolysis. Immune cell subset analysis of splenocytes by flow cytometry was also performed.
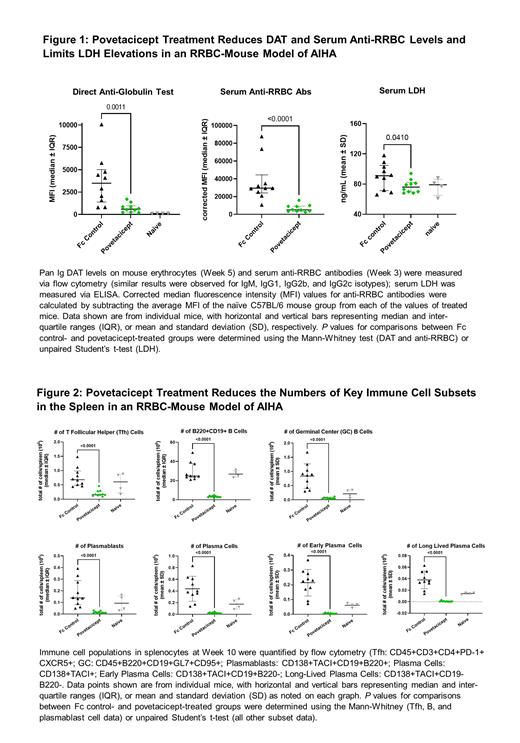
Results: As compared to Fc control, povetacicept treatment significantly reduced levels of anti-RRBC and DAT of IgM and IgG isotypes and significantly limited serum LDH elevations at various timepoints during the study ( Figure 1). Furthermore, at 10 weeks, numbers of T follicular helper (Tfh) cells, total B cells, germinal center (GC) B cells, and plasma cells were significantly lower in spleens of povetacicept-treated mice ( Figure 2).
Conclusions: Povetacicept effectively reduced pathogenic autoantibodies and key B cell populations (e.g., ASC) and limited serum LDH elevations in this mouse model of erythrocyte autoimmunity. These data suggest that povetacicept may reduce multiple pathogenic inflammatory processes including autoantibody production and may be an effective treatment for AIHA and other autoimmune cytopenias. Povetacicept is currently being evaluated in an open-label study (RUBY-4; NCT05757570) of adults with autoimmune cytopenias (ITP, AIHA, and CAD).
Disclosures
Lewis:Alpine Immune Sciences, Inc.: Current Employment, Current equity holder in publicly-traded company. Griffin:Alpine Immune Sciences, Inc.: Current Employment, Current equity holder in publicly-traded company. Maria:Alpine Immune Sciences, Inc.: Current Employment, Current equity holder in publicly-traded company. Enstrom:Alpine Immune Sciences, Inc.: Current Employment, Current equity holder in publicly-traded company; Tempest Therapeutics: Current equity holder in publicly-traded company, Ended employment in the past 24 months. Bankhead:Alpine Immune Sciences, Inc.: Current Employment, Current equity holder in publicly-traded company. Peng:Alpine Immune Sciences, Inc.: Current Employment, Current equity holder in publicly-traded company. Dillon:Alpine Immune Sciences, Inc.: Current Employment, Current equity holder in publicly-traded company, Patents & Royalties: Alpine Immune Sciences, Inc..

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal